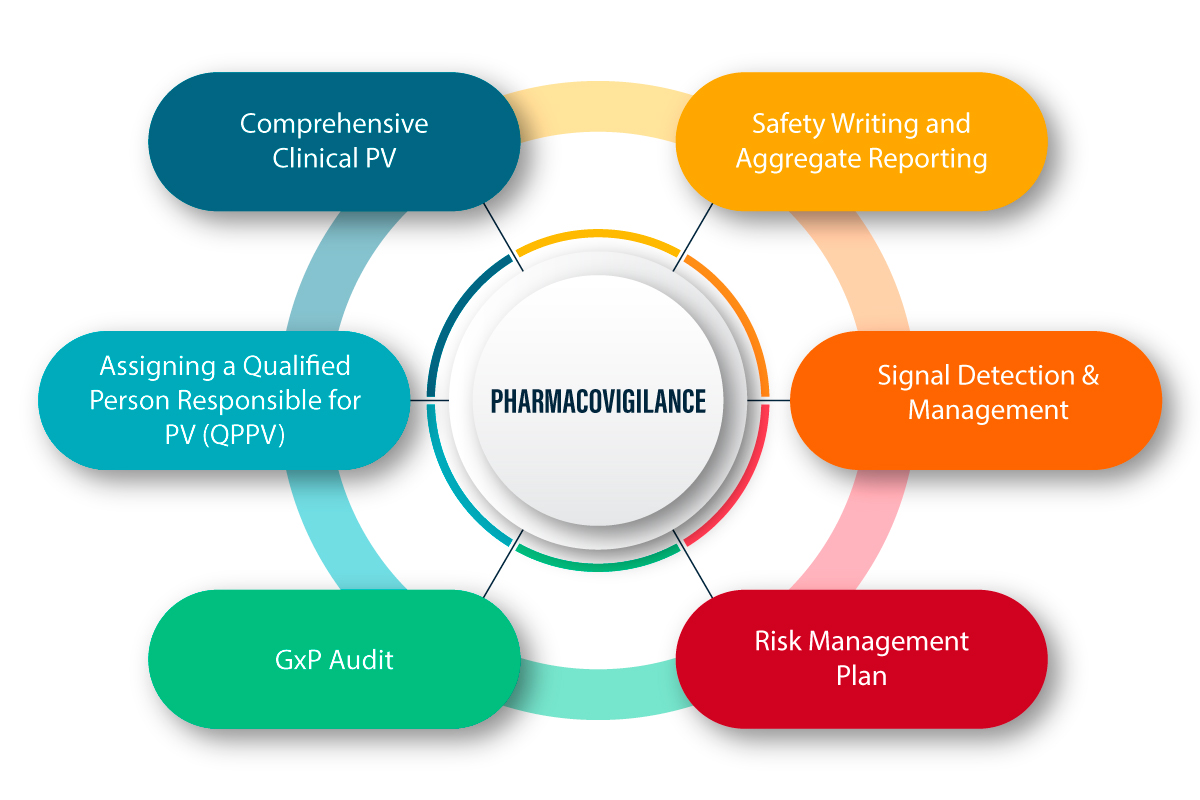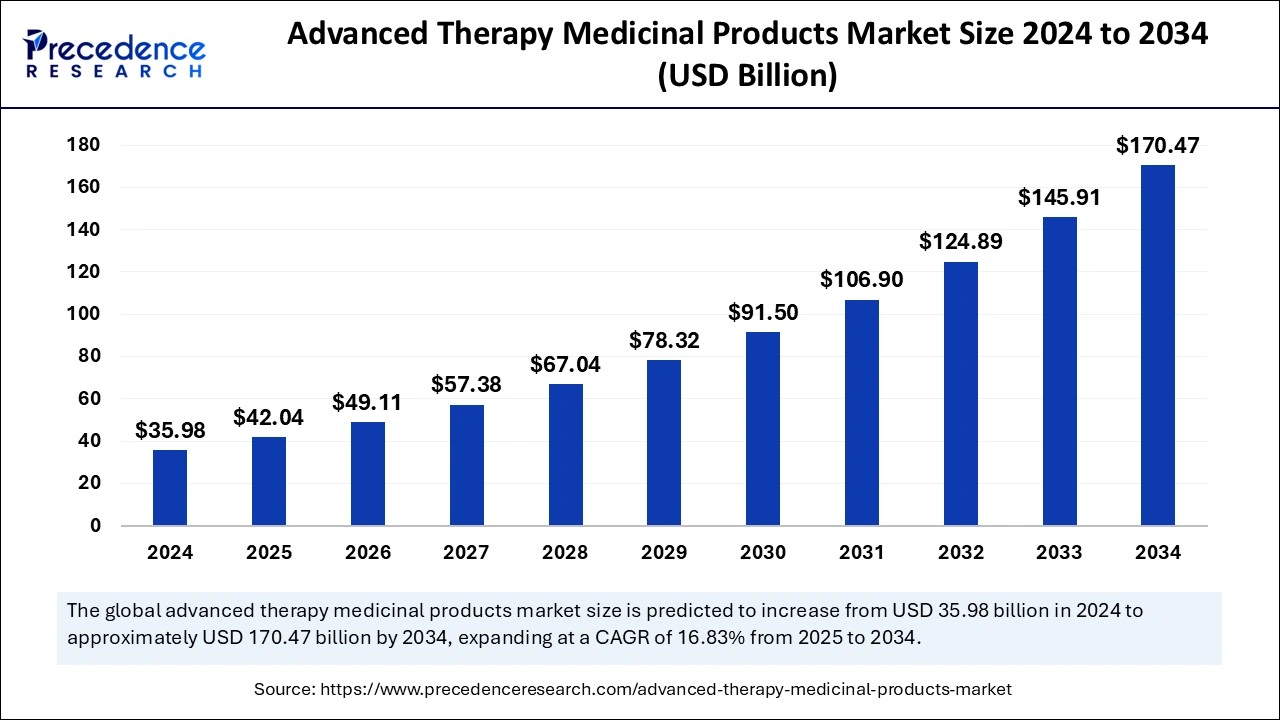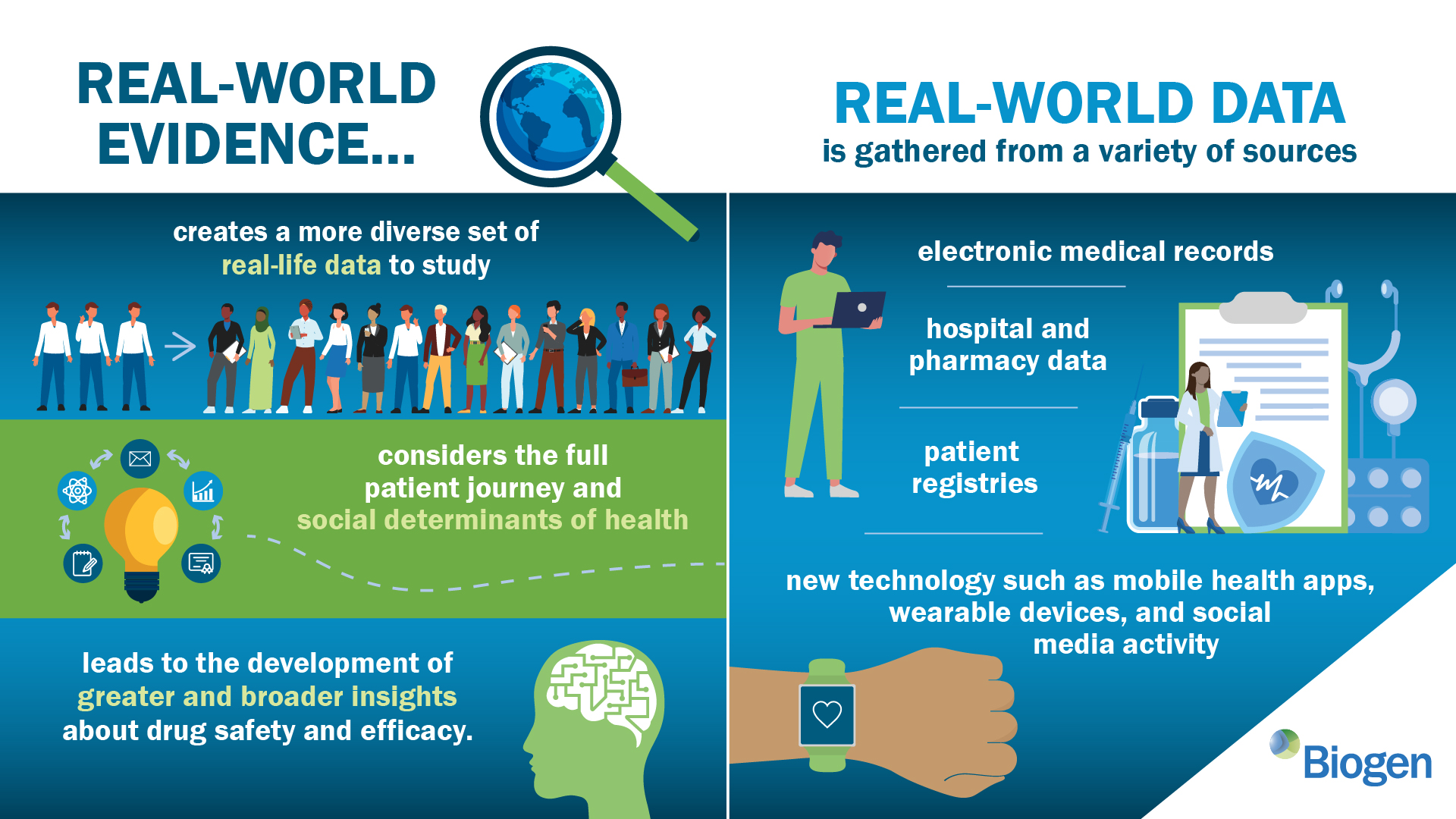
FDA Advisory Committee Recommends Approval for Novel Gene Therapy
The Cellular, Tissue and Gene Therapies Advisory Committee voted 9-3 in favor of recommending approval for a groundbreaking gene therapy targeting inherited retinal dystrophy, marking a significant milestone in ophthalmology.