FDA Advisory Committee Recommends Approval for Novel Gene Therapy
The Cellular, Tissue and Gene Therapies Advisory Committee voted 9-3 in favor of recommending approval for a groundbreak...
Read MoreNew guidance addresses manufacturing, quality controls, and clinical development considerations for cell and gene therapies entering European markets.

The European Medicines Agency (EMA) has released comprehensive updated guidelines for Advanced Therapy Medicinal Products (ATMPs), providing crucial clarity for developers of cell and gene therapies seeking European market authorization.
The updated guidelines, published on July 24, 2025, address several critical areas that have evolved significantly since the previous version. These changes reflect the agency's growing experience with ATMP applications and the need for more specific regulatory guidance.
Manufacturing and Quality Controls:
Our EU regulatory team successfully guided a CAR-T therapy through EMA's updated ATMP guidelines, addressing manufacturing comparability challenges and securing conditional approval 6 months ahead of schedule. With former EMA assessors on our team, we provide insider insights into European advanced therapy regulations.
Discover our comprehensive EMA ATMP regulatory servicesThe guidance provides updated recommendations for clinical trial design, particularly addressing unique challenges in ATMP development:
The updated guidelines emphasize the importance of robust quality management systems throughout the product lifecycle:
Key Requirements:
The guidance provides important clarifications on the regulatory pathways available for ATMPs in the EU:
Companies with ATMPs currently in development or under regulatory review should carefully evaluate these updated guidelines to ensure compliance. The EMA has indicated that:
Industry stakeholders have generally welcomed the updated guidance, noting that the clarifications will help streamline development and regulatory pathways. Key industry responses include:
The EMA plans to host a series of workshops in Q4 2025 to help industry stakeholders understand and implement the new requirements effectively.
Navigate complex EMA guidelines with our expert EU regulatory team specializing in advanced therapy medicinal products.
Learn MoreOur EU Regulatory Team consists of experienced regulatory professionals with deep expertise in pharmaceutical development and regulatory strategy.
Subscribe to our regulatory intelligence newsletter and receive curated updates, analysis, and insights delivered directly to your inbox.
Join 5,000+ regulatory professionals who trust our insights
Daily Intelligence
Expert Analysis
Strategic Insights

The Cellular, Tissue and Gene Therapies Advisory Committee voted 9-3 in favor of recommending approval for a groundbreak...
Read More
British regulatory authority grants breakthrough therapy designation to innovative monoclonal antibody targeting amyloid...
Read More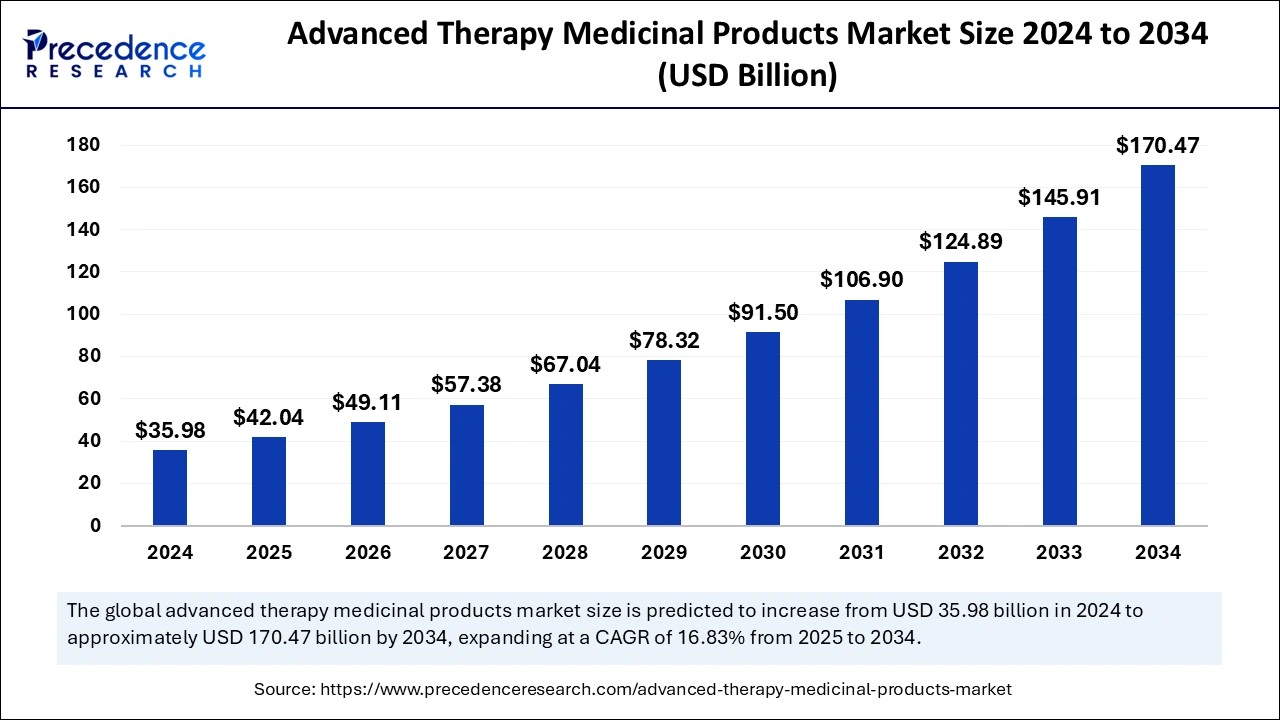
International Council for Harmonisation publishes comprehensive guidance on statistical methodologies for clinical trial...
Read More