FDA Advisory Committee Recommends Approval for Novel Gene Therapy
The Cellular, Tissue and Gene Therapies Advisory Committee voted 9-3 in favor of recommending approval for a groundbreak...
Read MoreKey discussions on emerging trends and regulatory challenges in advanced therapy development.

The European Medicines Agency's Scientific Advisory Group on Advanced Therapies (SAG-AT) will convene in Amsterdam on August 22, 2025, to address pressing scientific and regulatory challenges in ATMP development.
The two-day meeting will feature discussions on cutting-edge scientific developments and their regulatory implications:
Advance Regulatory Consulting has successfully supported over 200+ pharmaceutical and biotech companies in navigating complex regulatory pathways. Our team of former health authority experts provides strategic guidance from early development through market authorization and beyond.
Partner with regulatory experts who deliver resultsThe meeting will address several complex scientific and regulatory questions:
Strategic guidance for EMA scientific advice procedures and Scientific Advisory Group interactions.
Learn MoreOur EU Regulatory Team consists of experienced regulatory professionals with deep expertise in pharmaceutical development and regulatory strategy.
Subscribe to our regulatory intelligence newsletter and receive curated updates, analysis, and insights delivered directly to your inbox.
Join 5,000+ regulatory professionals who trust our insights
Daily Intelligence
Expert Analysis
Strategic Insights

The Cellular, Tissue and Gene Therapies Advisory Committee voted 9-3 in favor of recommending approval for a groundbreak...
Read More
New guidance addresses manufacturing, quality controls, and clinical development considerations for cell and gene therap...
Read More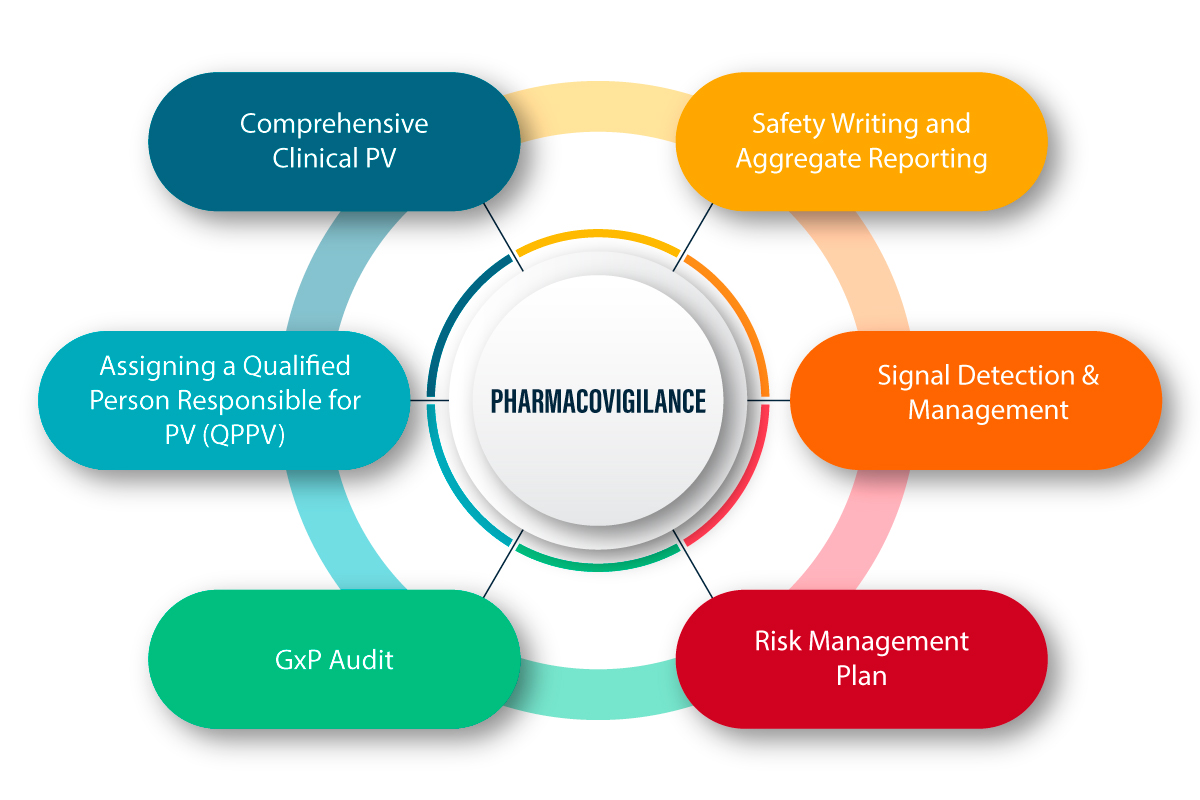
British regulatory authority grants breakthrough therapy designation to innovative monoclonal antibody targeting amyloid...
Read More