FDA Advisory Committee Recommends Approval for Novel Gene Therapy
The Cellular, Tissue and Gene Therapies Advisory Committee voted 9-3 in favor of recommending approval for a groundbreak...
Read MoreComprehensive overview of the updated EU pharmaceutical legislation and its implications for drug development and market authorization processes.

The European Union has implemented sweeping pharmaceutical legislation reforms in 2025, fundamentally changing the regulatory landscape for drug development, approval, and market access across all 27 member states.
The reformed legislation, officially known as the "EU Pharmaceutical Package 2025," represents the most significant overhaul of European pharmaceutical law in over two decades. The package includes revisions to both the Pharmaceutical Directive and Regulation, addressing modern challenges in drug development and patient access.
Key Legislative Components:
Advance Regulatory Consulting has successfully supported over 200+ pharmaceutical and biotech companies in navigating complex regulatory pathways. Our team of former health authority experts provides strategic guidance from early development through market authorization and beyond.
Partner with regulatory experts who deliver resultsOne of the most significant changes involves the integration of digital health technologies into the regulatory framework:
The reformed legislation introduces several mechanisms to expedite patient access to innovative treatments:
New Accelerated Pathways:
The legislation includes a comprehensive review of the incentive system for pharmaceutical innovation:
In response to lessons learned from recent global health challenges, the reform includes robust supply chain provisions:
The reformed legislation incorporates environmental considerations throughout the pharmaceutical lifecycle:
Sustainability Requirements:
The reform significantly increases transparency and patient participation in the regulatory process:
The European Commission has established a phased implementation approach:
Key Implementation Milestones:
The reformed legislation will require significant operational adjustments across the pharmaceutical industry:
The EU reforms are expected to influence regulatory practices worldwide:
Navigate the complex new EU pharmaceutical legislation with our expert regulatory team's strategic guidance and implementation support.
Learn MoreOur EU Regulatory Team consists of experienced regulatory professionals with deep expertise in pharmaceutical development and regulatory strategy.
Subscribe to our regulatory intelligence newsletter and receive curated updates, analysis, and insights delivered directly to your inbox.
Join 5,000+ regulatory professionals who trust our insights
Daily Intelligence
Expert Analysis
Strategic Insights

The Cellular, Tissue and Gene Therapies Advisory Committee voted 9-3 in favor of recommending approval for a groundbreak...
Read More
New guidance addresses manufacturing, quality controls, and clinical development considerations for cell and gene therap...
Read More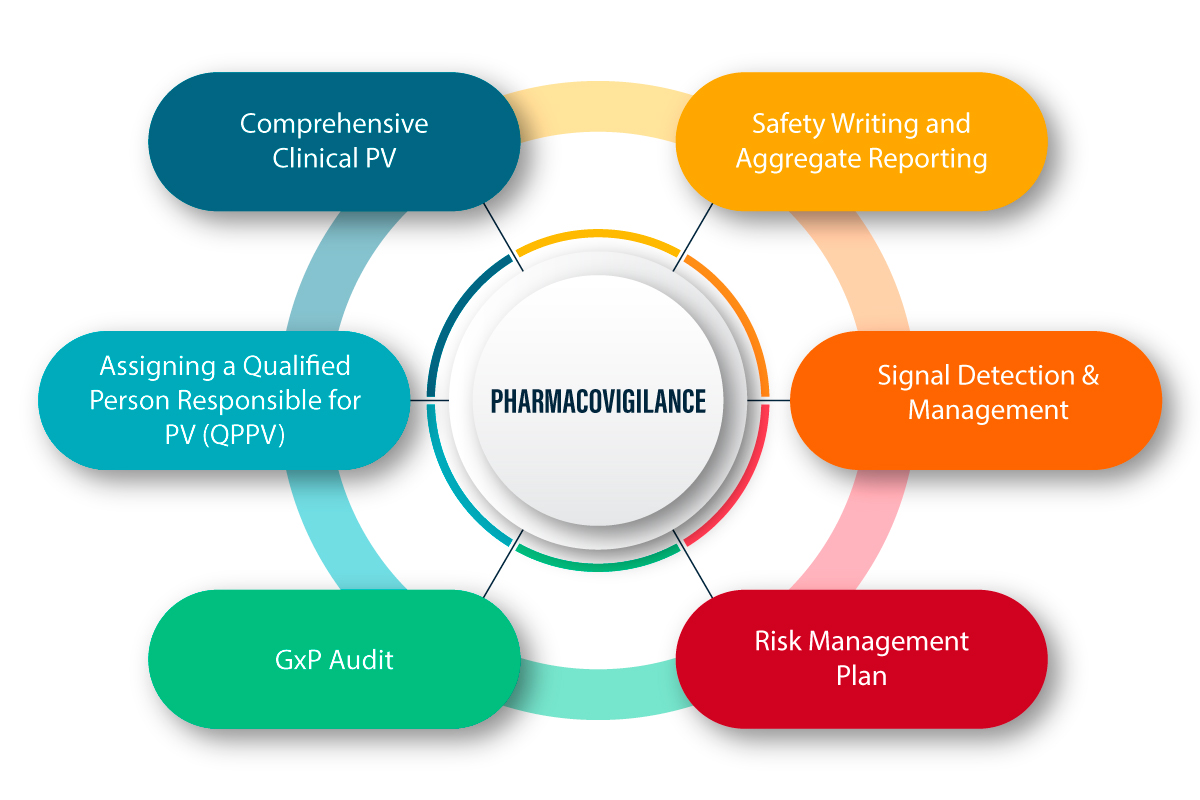
British regulatory authority grants breakthrough therapy designation to innovative monoclonal antibody targeting amyloid...
Read More