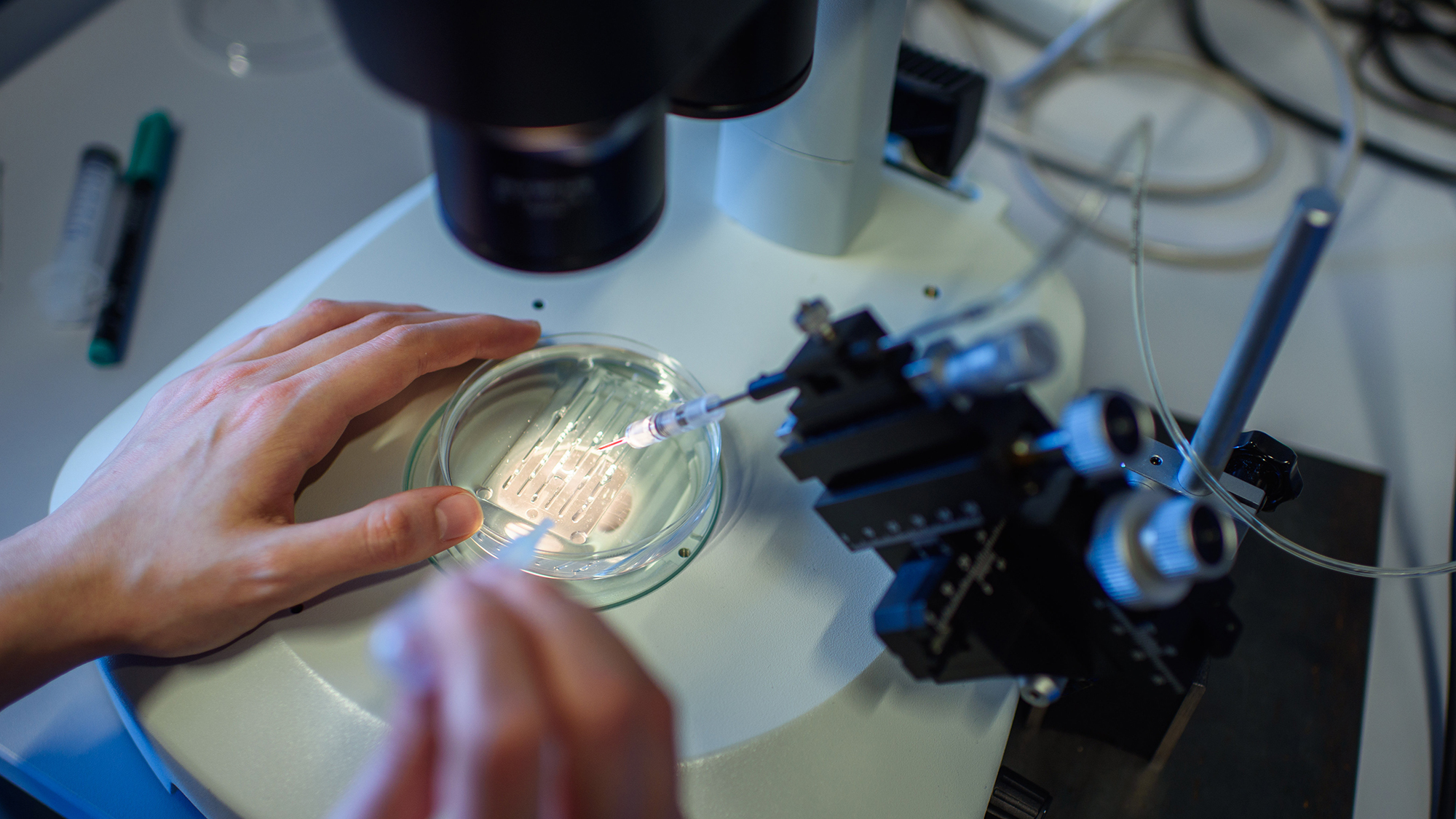
FDA Advisory Committee Recommends Approval for Novel Gene Therapy
The Cellular, Tissue and Gene Therapies Advisory Committee voted 9-3 in favor of recommending approval for a groundbreak...
Read MoreInternational Council for Harmonisation publishes comprehensive guidance on statistical methodologies for clinical trials in pharmaceutical development.
The International Council for Harmonisation (ICH) has released the highly anticipated E9(R1) guidance document on Statistical Analysis Plans (SAPs), providing updated recommendations for statistical methodologies in clinical trials across the pharmaceutical industry.
The new guidance represents a significant evolution from the original ICH E9 guideline, incorporating modern statistical approaches and methodologies that have emerged over the past two decades of clinical research.
Key Areas of Update:
Advance Regulatory Consulting has successfully supported over 200+ pharmaceutical and biotech companies in navigating complex regulatory pathways. Our team of former health authority experts provides strategic guidance from early development through market authorization and beyond.
Partner with regulatory experts who deliver resultsA central focus of the updated guidance is the practical implementation of the estimand framework, which provides a structured approach to defining what is to be estimated in clinical trials:
The guidance provides detailed recommendations for handling intercurrent events, which have historically posed challenges in clinical trial analysis:
Strategic Approaches:
The updated guidance embraces modern statistical approaches that have gained acceptance in regulatory submissions:
The ICH has established a phased implementation timeline to allow industry adaptation:
Key Implementation Dates:
Major regulatory authorities have indicated strong support for the updated guidance:
To ensure successful implementation, industry stakeholders should consider:
Leading biostatisticians have praised the guidance as a significant step forward in clinical trial methodology. The emphasis on clear estimand definition is expected to:
Our expert biostatisticians provide comprehensive support for ICH E9(R1) implementation, estimand framework development, and statistical analysis planning.
Learn MoreOur Biostatistics Team consists of experienced regulatory professionals with deep expertise in pharmaceutical development and regulatory strategy.
Subscribe to our regulatory intelligence newsletter and receive curated updates, analysis, and insights delivered directly to your inbox.
Join 5,000+ regulatory professionals who trust our insights
Daily Intelligence
Expert Analysis
Strategic Insights

The Cellular, Tissue and Gene Therapies Advisory Committee voted 9-3 in favor of recommending approval for a groundbreak...
Read More
New guidance addresses manufacturing, quality controls, and clinical development considerations for cell and gene therap...
Read More
British regulatory authority grants breakthrough therapy designation to innovative monoclonal antibody targeting amyloid...
Read More