FDA Advisory Committee Recommends Approval for Novel Gene Therapy
The Cellular, Tissue and Gene Therapies Advisory Committee voted 9-3 in favor of recommending approval for a groundbreak...
Read MoreEnhanced pharmacovigilance requirements for advanced therapy medicinal products.
The European Medicines Agency (EMA) has released comprehensive updates to pharmacovigilance requirements specifically targeting advanced therapy medicinal products (ATMPs), reflecting the unique safety monitoring challenges posed by cell and gene therapies.
The updated guidelines address critical gaps in safety monitoring for ATMPs that have become apparent as more of these complex therapies reach the market. The enhanced requirements reflect the long-term nature of potential adverse effects and the need for specialized monitoring approaches.
Products Covered by Updated Guidelines:
Advance Regulatory Consulting has successfully supported over 200+ pharmaceutical and biotech companies in navigating complex regulatory pathways. Our team of former health authority experts provides strategic guidance from early development through market authorization and beyond.
Partner with regulatory experts who deliver resultsThe updated guidelines introduce more granular reporting requirements for ATMP-specific adverse events:
The guidelines require comprehensive Risk Management Plans (RMPs) tailored to ATMP characteristics:
Enhanced RMP Components:
ATMPs require modified PSUR formats to accommodate their unique safety profiles:
The updated guidelines emphasize proactive signal detection approaches:
Enhanced Signal Detection Requirements:
The guidelines establish new standards for communicating ATMP safety information:
Updated requirements emphasize active patient participation in safety monitoring:
The guidelines mandate advanced data management capabilities:
Technology Requirements:
Enhanced collaboration requirements between regulatory authorities:
The EMA has established a phased implementation approach:
Key Implementation Milestones:
Companies must undertake comprehensive preparation activities:
The enhanced requirements will require significant resource investments:
However, the EMA emphasizes that these investments are essential for ensuring patient safety and maintaining public confidence in advanced therapies.
Comprehensive pharmacovigilance support for advanced therapy medicinal products with specialized expertise in long-term safety monitoring.
Learn MoreOur Pharmacovigilance Team consists of experienced regulatory professionals with deep expertise in pharmaceutical development and regulatory strategy.
Subscribe to our regulatory intelligence newsletter and receive curated updates, analysis, and insights delivered directly to your inbox.
Join 5,000+ regulatory professionals who trust our insights
Daily Intelligence
Expert Analysis
Strategic Insights

The Cellular, Tissue and Gene Therapies Advisory Committee voted 9-3 in favor of recommending approval for a groundbreak...
Read More
New guidance addresses manufacturing, quality controls, and clinical development considerations for cell and gene therap...
Read More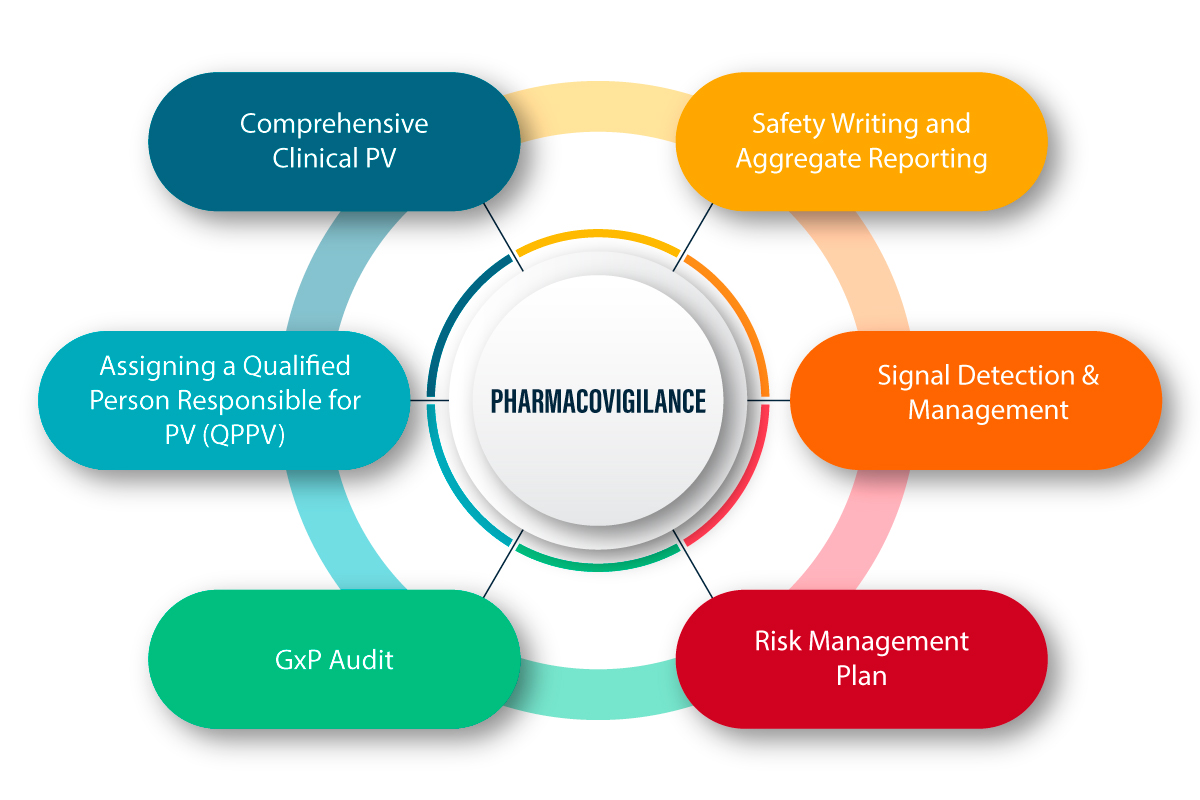
British regulatory authority grants breakthrough therapy designation to innovative monoclonal antibody targeting amyloid...
Read More